There are three types of “rays” that are discussed in nuclear physics:
- alpha (α) rays
- beta (β) rays
- gamma (γ) rays

Alpha rays
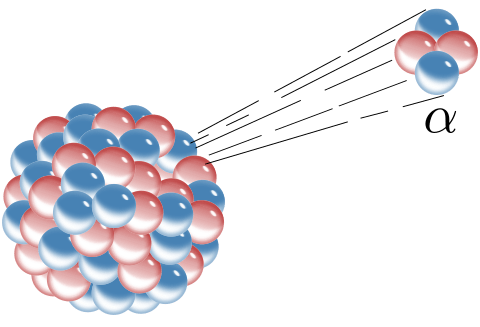
An alpha ray is emitted from a heavy nucleus during a process called alpha decay. An alpha ray is a particle made of two protons and two neutrons; this turns out to be a helium nucleus.
In fact, helium (like in helium balloons) is found in pockets underground. It is created through alpha decay from radioactive elements within the earth.
Beta rays
A beta ray is an electron emitted from a heavy nucleus during a process called beta decay.
(If a positron is emitted instead of an electron, the process is called β+ decay, since a positron has a positive charge.)

Gamma rays
Unlike alpha rays and beta rays, gamma rays are not particles. They are extremely energetic photons of electromagnetic radiation.
Comparison
| Ray | Symbol | Charge | Composition | Danger |
| alpha | α | +2 | Helium nucleus | Does not penetrate skin; dangerous if swallowed |
| beta | β– | -1 | Electron | Does penetrate skin |
| gamma | γ | 0 | Photon | Most dangerous; difficult to shield against |

Leave a comment