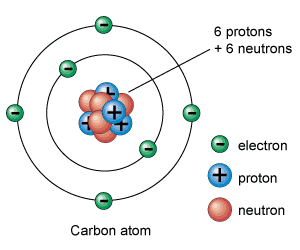
An atom is the smallest unit of an element.
The atom has a nucleus at the center with electrons surrounding it.
The nucleus is made up of protons and neutrons. Because they are found in the nucleus, protons and neutrons are called nucleons.
The electrons are organized into shells or orbitals around the nucleus.
The atomic number of the atom is equal to the number of protons in the nucleus. The atomic number is represented by Z.
The atomic mass of the atom is equal to the number of protons and neutrons in the nucleus. The atomic mass is represented by A.
Leave a comment