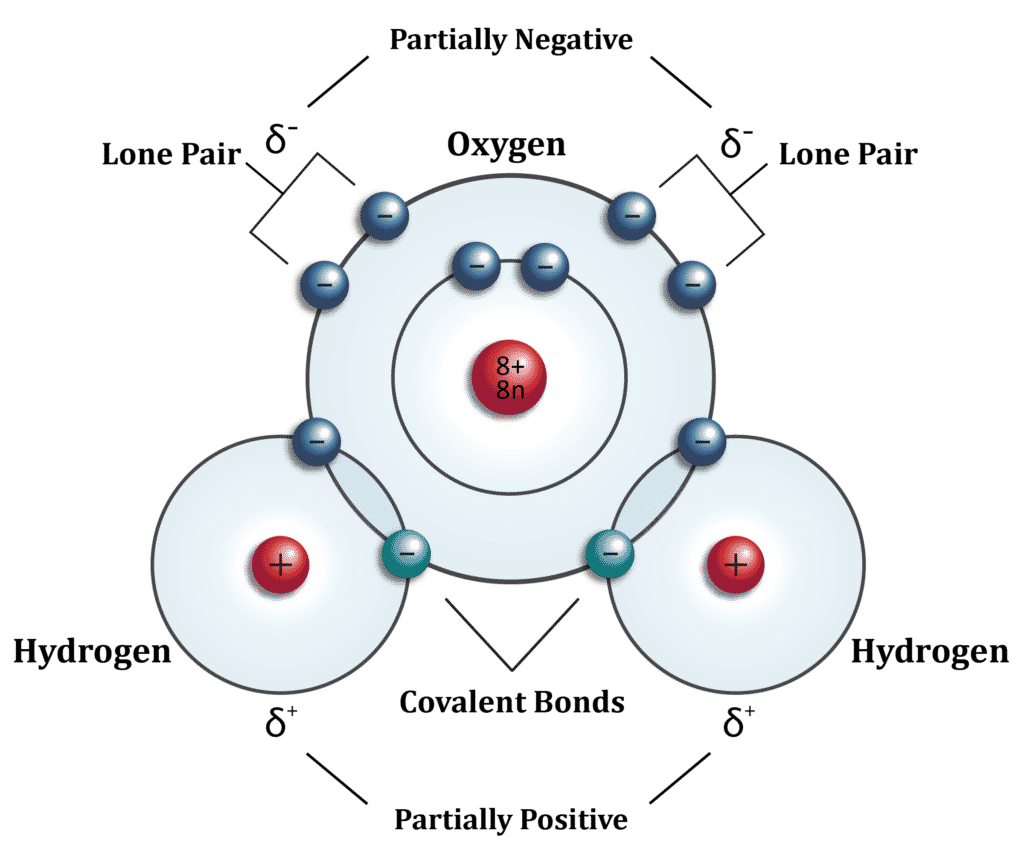
What molecule is this?
This is water, a compound of one oxygen and two hydrogen atoms.
What do the red circles represent?
They represent the nuclei. You could say that they represent the protons in the hydrogen atoms since the nucleus of a hydrogen atom is composed of a single proton. However, it is more consistent to interpret the red circles as being nuclei in all of the atoms.
What do the blue circles represent?
Electrons
What does the 8+ and 8n represent?
The 8 protons (which are positively charged) and 8 neutrons (which are neutral) found in the nucleus of an oxygen atom. It is important to recognize that the 8 protons would tell you that this is an oxygen atom even if it were not labeled as oxygen. It is also important to recognize that, although the most common isotope of oxygen has 8 neutrons, there are other isotopes of oxygen which have more or fewer neutrons.
Why are there two blue circles around each of the hydrogens?
A hydrogen atom has only one electron, making the atom electrically neutral. The hydrogen is sharing electrons with the oxygen atom to fill up its electron shell. Because the two electrons are being shared by the oxygen and hydrogen, the hydrogen continues to have the equivalent of one electron and remains neutral.
Why are there two blue circles on the inner circle and 8 blue circles on the outer circle around the oxygen?
The electron shell closest to the nucleus can only hold two electrons. The second electron shell can hold up to 8 electrons. The electron structure is most stable when the shell is filled. Therefore, there are 10 electrons around the oxygen atom even though it only needs 8 to be electrically neutral, but again, because 4 of those electrons are shared with the hydrogens, the oxygen atom remains electrically neutral.
What is the label “covalent bonds” indicating?
This is the bond formed between the atoms that share electrons.
What is a “lone pair”?
Electrons fill an orbital by first filling 4 states. Additional electrons then begin pairing up with these first 4. Pairs of electrons that are not shared with other atoms are known as “lone pairs”.
What does δ+ and δ– indicate?
The Greek symbol δ is a lower-case delta. Because a river delta constantly changes, Δ and δ have come to be associated with the concepts of “change” and “difference”.
In this case, δ+ and δ– indicate a slightly more positive or negative region, respectively.
How does this diagram explain the offset angle of the hydrogen atoms in a water molecule?
Rather than being directly across from each other, the hydrogen atoms are pushed off to the side by the lone pairs of electrons.
Why couldn’t the one hydrogen atom be directly across from the other hydrogen atom?
This diagram is a 2-dimensional representation of a 3-dimensional molecule. In actuality, the 4 sets of electron pairs (2 lone pairs and 2 shared pairs) are located at the four corners of a pyramid.
Leave a comment