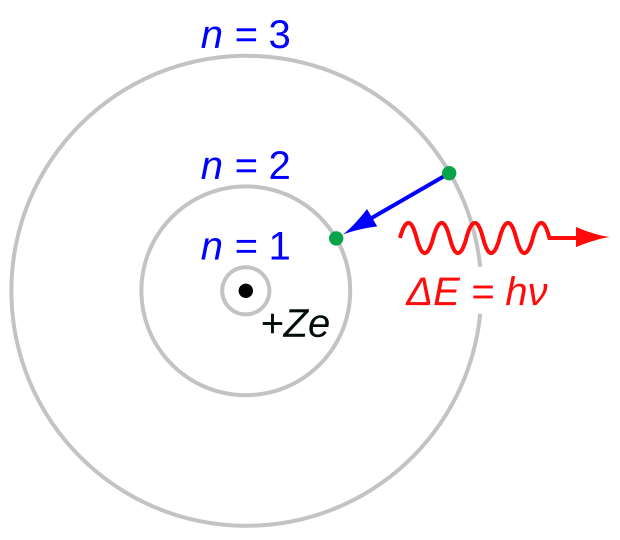
What is this?
A model of an atom
Who was the scientist who came up with this?
This is the Bohr model of an atom, named after Niels Bohr.
What does the black dot in the center represent?
The nucleus
What do the green dots represent?
Both dots represent a single electron.
What do the gray rings represent?
They represent the electron shells around the nucleus.
What is the meaning of +Ze?
Z is the symbol for the atomic number, the number of protons in the nucleus. e is the symbol for the electric charge. The plus sign indicates that the charge on the nucleus is positive. Taken together, this indicates the total charge on the nucleus, which is positive and equal to the number of protons in the nucleus multiplied by the charge of each proton (which is the same as the charge on an electron).
What does the blue arrow represent?
The blue arrow represents the transition of an electron moving from a higher shell to a lower shell. Equivalently, it represents the transition of an electron from a higher energy state to a lower energy state. This transition is what is referred to as a “quantum leap”.
What does the red squiggly line represent?
A photon of light being released by the atom as the electron makes this transition.
What are the symbols in the red equation and what do they mean?
E is energy.
Δ is the uppercase Greek delta, which frequently represents change or difference. In this case, it is indicating the difference in the energy levels of the electron, or the loss of energy from the atom as the photon leaves.
h is Planck’s constant.
ν is not a Latin v. It is a lowercase Greek nu. It is commonly used to represent frequency.
Taken together, the equation indicates that the energy of a photon is proportional to its frequency and the equation can be used to calculate the energy of a photon given its frequency or the frequency of a photon produced by a given amount of energy.
What do the equations in blue represent?
The number of the electron shell. n =1 is the electron shell closest to the nucleus.
n is the first quantum number.
The other quantum numbers commonly discussed in chemistry include l, ml, and ms.
What can you say about what element this diagram is illustrating?
Not much. The illustration is not meant to depict a specific element, which is why the nucleus is shown to have a +Ze charge.
However, it is very difficult for an electron to jump from the third shell to the second shell in a larger atom because the second shell is already filled. It could certainly be neon, which has a completely filled second shell. In that case, the electron would have come from the second shell initially (by absorbing a photon) and is now jumping back down by releasing a similar photon.
It would almost certainly not be uranium, for instance, which has its first four shells completely filled, and therefore no room for an electron to move between its third and second shells.
The single electron of hydrogen is normally thought of as being in the first shell. But it is possible for that electron to be in higher shells when it is in an excited state.

Leave a comment