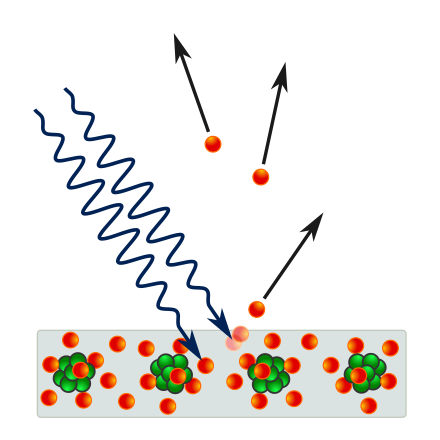
What do the two squiggly lines represent?
They represent light.
What do the green clumps represent?
They represent the nuclei of atoms. Each green circle represents a nucleon, either a proton or neutron. In this diagram, the distinction is unimportant.
What do the red circles represent?
The red circles represent electrons.
What do the straight arrows represent?
They represent the movement and direction of movement of the electrons.
What does the gray rectangle represent?
The gray rectangle represents a block of solid material which contains the atoms.
What do the faded red balls represent?
The faded red balls represent the former positions of the electron before it left the block of material.
Why is it hard to associate specific red balls with specific green clumps?
The orbitals of the electrons are not shown, and, in fact, it is hard to tell which electrons go with which nuclei because this material is a metal, and the electrons are shared between the atoms. This is referred to as a “sea of electrons” and it is the reason that metals conduct electricity easily.
What effect is this diagram illustrating?
This diagram is showing the photoelectric effect, in which photons (the squiggly lines) strike a piece of metal (the gray rectangle) and knock electrons (the red circles) off the material. This effect was explained by Albert Einstein as the first proof of the existence of photons. He won the Nobel Prize for this explanation.

Leave a comment