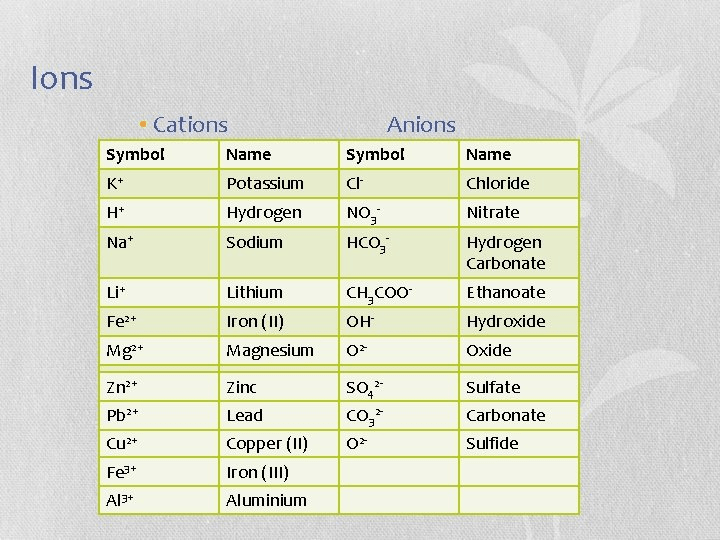
What is this?
It’s a table of ions.
What is an ion?
It is a charged atom or combination of atoms. It gets charged by gaining or losing electrons so that the negatively charged electrons and the positively charged protons no longer balance.
What do the superscripted numbers represent?
They are the charges on the ions.
What is the difference between a cation and an anion?
Cations are positively charged and anions are negatively charged.
Which ions are missing an electron?
The cations, which are positively charged.
Why is potassium listed with the element’s name while chlorine is listed as chloride?
This is the naming convention for positive and negative ions.
What is the difference between Iron (II) and Iron (III)?
These are different oxidation states of iron.
Why is the chemical symbol for lead Pb?
The symbol comes from its Latin name, plumbus, which is where we get the word plumber because pipes used to be made out of lead.
What do the ions that end in “-ate” have in common?
They all contain oxygen.
What about this table is incorrect?
Clearly, a sulfide ion should have a sulfur atom in it. Its chemical formula should not be the same as oxide.
What are the ions with more than one atom called?
polyatomic ions
Why are all of the polyatomic ions listed as anions?
There are relatively few polyatomic cations. The only one that is commonly known is ammonium, NH4+
Where are potasium, hydrogen, sodium, and lithium on the periodic table?
They are all in the first group, which is the first column on the left. From top to bottom, they are hydrogen, lithium, sodium and potassium.
What is the chemical formula for magnesium chloride?
MgCl2
What is the chemical formula for lithium chloride?
LiCl
What is the chemical formula for zinc ethanoate?
Zn(CH3COO)2
Goals of this image:
- Test your knowledge of basic chemistry
- What an ion is
- Naming conventions
- Oxidation states
- Group I elements
- Recognizing patterns and discrepancies
- Cations are positive, anions are negative
- -ate indicates a polyatomic ion containing oxygen
- Sulfide is listed incorrectly
- Polyatomic ions
- Recognizing that most polyatomic ions are negatively charged
- Creating proper chemical formulas
- Interpreting chemical names
- Combining ions in proper ratios based on their charges
Leave a comment