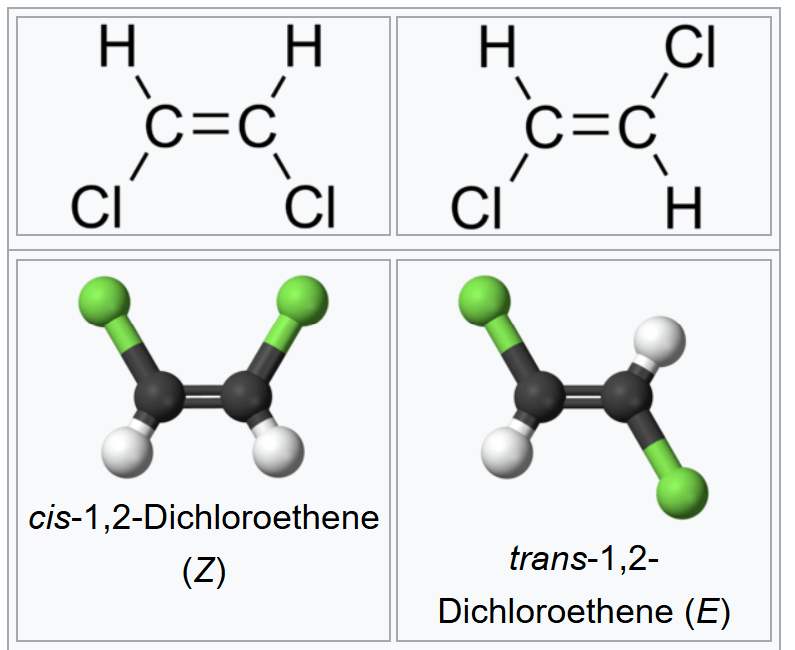
What is this?
Diagrams of organic molecules
What kind of diagrams are the lower images?
These are called ball-and-stick models.
What is the relationship between the diagrams at the top and the diagrams on the bottom?
They represent the same molecule.
What is the relationship between the diagrams on the left and on the right?
These are isomers. They have the same atoms in the same proportions (and therefore, the same chemical formula), but the attachments are different.
What does the green represent?
chlorine is traditional colored green because it forms a gas that is lightly tinted green.
What does the white represent?
hydrogen
The images on the top and bottom are flipped, although they still represent the same molecule.
What is the chemical formula for these molecules?
Both have a chemical formula of C2H2Cl2.
Why is the double bond between the carbons important?
The double bond does not allow the carbons to rotate. If there were a single bond between the carbons, then the carbons could rotate relative to each other and there would not be two different isomers because one could transform into the other.
What do the prefixes cis- and trans- indicate?
Whether the chlorines are on the same side of the double bond (cis) or the opposite sides of the double bond (trans).
Why is the bond between the chlorine and carbon longer than between the hydrogen and carbon?
The chlorine atom is significantly larger than the hydrogen atom. The bond is the length between the nuclei which are necessarily farther away in the case of chlorine.
What is the chemical formula for ethene?
C2H4
Goals of this image:
- Infer the relationships between the diagrams
- Interpret a ball-and-stick model
- Colors
- Lengths of bonds
- Understand isomers
- Why the double bond is important
- Same chemical formula but different connections
- Understand naming of chemicals
- Recognize these are formed by substitution on an ethene
- Infer structure of ethene
- Infer the meaning of cis and trans
Leave a comment