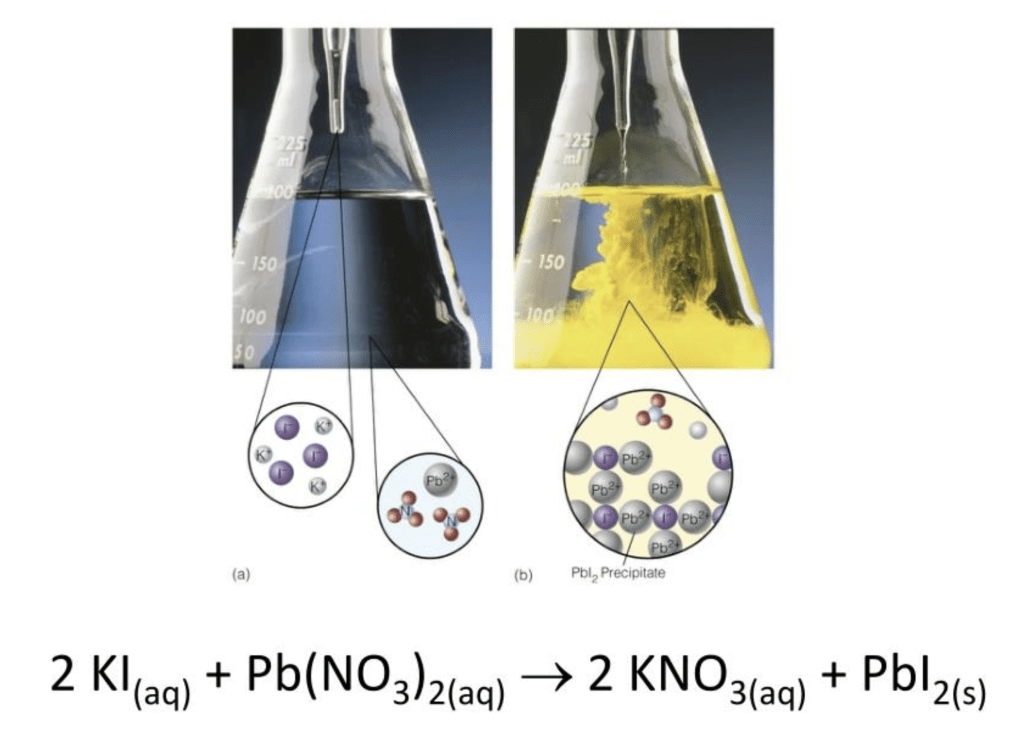
What glassware is shown?
Erlenmeyer flasks and pipettes
What are the names of the chemicals involved?
| KI | Potassium Iodide |
| Pb(NO3)2 | Lead Nitrate |
| KNO3 | Potassium Nitrate |
| PbI2 | Lead Iodide |
Is the chemical equation balanced?
Yes
How many oxygens are shown in the reaction?
6
What type of chemical reaction is this?
It is called a double replacement reaction.
What do (aq) and (s) mean?
(aq) means the chemical is in a solution with water. (s) means the chemical is a solid.
What color are each of the chemicals involved?
They are all clear except for lead iodide which is yellow.
In general, what are the chemicals on the left side and the right side of the chemical equation called?
The chemicals on the left are called the reactants.
The chemicals on the right are called the products.
What kind of bonds are in these chemicals?
The bonds between the nitrogen and oxygens in the nitrate ion are covalent bonds. The other bonds are all ionic.
Knowing that the objects in the circles in (a) must correspond to the chemicals on the left side of the equation, how can you determine what corresponds to what without zooming in enough to see the letters?
The circle on the left contains small white circles and larger purple circles. Iodine is somewhat purplish and is often represented by purple circles. Iodine atoms are larger than potassium atoms.
The circle on the right contains a large gray circle and clumps of 4 atoms. The large gray circle represents lead and the clumps are the nitrate ions.
What are the charges on each of the ions involved?
| Ion | Charge |
| K | +1 |
| I | -1 |
| NO3 | -1 |
| Pb | +2 |
You should recognize that potassium is in the first column on the periodic table and therefore has a +1 charge. From there, you can determine the charges on the other ions by how many ions it takes to combine with the others.
What part of this image is incorrect?
The precipitate doesn’t seem to be drawn correctly. There should be twice as many iodine ions as there are lead ions.
Goals of this image:
- Interpret a chemical equation
- reactants and products involved
- determining if it is balanced
- understanding symbols for what state the chemical is in
- recognizing double replacement reactions
- determine the ionic charges
- converting chemical formulas into names of chemicals
- Matching the corresponding parts of the image
- Determining what is inconsistent or inaccurate about an image
Leave a comment